Adiabatic cooling / adiabatic humidification
Calculator
Pressure: |
| Point A | Point B | Increase | |
|---|---|---|---|
| Relative Humidity (%) |
|||
| Temperature (ÂșC) | |||
| Water per volume (g/m3) |
g of water per kg of dry air (g/kg) |
Enthalpy (kJ/kg) | |
For a more detailed calculation go to the online psychrometric diagram.
What is adiabatic humidification or cooling?
In an adiabatic thermodynamic process there is no exchange of heat between the system and its surroundings. In our case the temperature change is produced by the evaporation of water, not from an external source of heat. Evaporation of water needs energy, and it comes from the air that will reduce its temperature. So, at the same time, humidification and cooling is produced.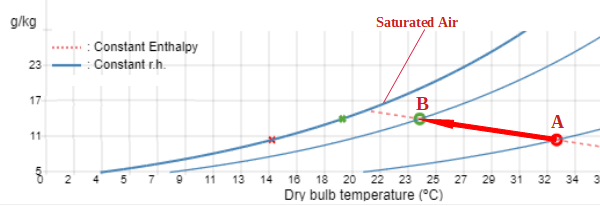
As there is no exchange of heat, the enthalpy will not change, so the point moves from A to B along the constant enthalpy line on the psychrometric chart. Note that the constant enthalpy and the constant wet bulb line on a psychrometric diagram are almost parallel. This means that wet bulb will remain almost constant as well.
There are several technologies to produce adiabatic humidification by introducing water into air, some of them works dispersing water droplets and others let water evaporate from a wetted media. In either case, the temperature will decrease and the relative humidity will increase. Examples of adiabatic cooling or humidifying are: high pressure fogging systems, ultrasonic humidifiers and wetted evaporative media.